A Laboratory Must Have A Written Quality Assurance Policy That
We came up with a list of laboratory quality assurance plan examples in PDF that you can use as references for the processes that you will immerse in if you want to make your own laboratory quality assurance plan. It is designed to be customizable to any.
The quality assurance QA officer notes that the records from mouse necropsies have not been copied for sending to the pathologist for a particular study.

A laboratory must have a written quality assurance policy that. A component of quality assurance program that focuses on ensuring accuracy in laboratory test results through careful monitoring of a test. Browse through and download these examples so you can have format and content guides that will allow you to. Quality ControlQuality Assurance QCQA can be defined as the set of planned and systematic activities focused on providing confidence that quality requirements will be fulfilled.
Method to evaluate the proper performance of testing procedures. The quality manager is the person most directly responsible for assuring that the quality policies and procedures are carried out. Laboratory data should be produced under a quality system.
Possible if there were no quality assurance programme. The laboratory must maintain a written current document control plan that addresses and ensures the following vital elements of SOPs. Laboratories at all levels national regional and local which conduct HIV testing should have a functioning QA programme.
Quality Assurance for a recreational water monitoring programme will apart from helping to ensure that the results obtained are corr ect increase the confidence of funding bodies and the public. Quality ControlQuality Assurance. Quality Assurance QA An evaluation of health care services as compared to accepted standards.
Assurance that all SOPs are procedurally accurate and relevant as well as review of each SOP at. 9 Security Strategic Plan Examples. MARLAP fully endorses the need for a laboratory quality system and a quality manual that delineates the quality assurance QA policies and QC practices of the.
91 Quality assurance Quality assurance QA refers to the full range of practices employed to ensure that laboratory results are reliable. This is a new requirement for ISO standards. Each laboratory or testing service conducting HIV testing should routinely monitor and assess the quality of the testing process in the preanalytical analytical and postanalytical phases.
ISO 15189 415 i states that a laboratory must have a quality manager. A laboratory must have a written quality assurance policy that. And there must be a mechanism for implementation and.
A master list of SOPs currently used in the laboratory. 8 Laboratory Quality Management System 1-1. However the results obtained for that 90 per cent will be accurate reliable and of consistent value to the monitoring programme.
Ad and Cookie Policy. There must be a strong supporting organizational structure management commitment is crucial. The importance of laboratory quality Laboratory quality can be de fi ned as accuracy r eliability and timeliness of reported test results.
The study directors response is that no abnormal effects were found in any of the four groups of mice so heshe felt it was not necessary to send the 40 pages of data to the pathologist. An authorization process that is standard and consistent limiting SOP approvals to laboratory management. 522 Communicating the Quality Policy.
Writing Quality Assurance Plans Conducting Written Quality Assurance Reviews An effective quality assurance QA plan addresses the entire laboratory process from the time a patient or sample arrives in your facility to the moment results are recorded on patient charts and reported to. Eligibility The laboratory must have a qualified laboratory director successfully participate in the appropriate proficiency testingexternal quality assurance and be performing patient testing. Current Good Manufacturing Practice cGMP Good Laboratory Practice GLP GCP etc and local.
He or she must be. Some staff may have specialized expertise in a limited area such as sample transport or housekeeping but could serve as auditors in these areas. 9 Health and Safety Strategic Plans.
CLIA 1992 mandates that there must be ___ policies and procedures for a comprehensive quality assurance program that will evaluate the ongoing and overall quality of the testing process written control solutions are chemicals similar to the testing specimen that have an ___ value. Quality Assurance Policy Statement It is the policy of Anatek Labs that there shall be sufficient quality assurance activities conducted to ensure that all. Of quality laboratories must adopt a systematic approach to the organization planning and review of their testing services.
Director Qualifications The CAP requires specific qualifications for the laboratory director the person responsible for operation of the laboratory. Quality Assurance Project Plans QAMS-005 80 and Guidance on Preparation of Laboratory Quality Assurance Plans EPA 910 9-92-0332. The quality assurance department must operate independently from the operational units and it must regularly perform quality review activities self-inspection auditsinternal audits to ensure compliance within operational units with Company quality standards good working practices GxPs.
Quality Assurance extends to all aspects of data collection from sanitary surveys to laboratory procedures. 1 that incorporates planning implementing and internal assessment of the work performed by the laboratory including QC. Standards policies and instructions estto ablish a credible laboratory quality assurance program.
ISO 90012015 requires the policy to be maintained as documented information refer to Clause 751aYou should check whether the quality policy has been applied throughout the organization and that the quality policy is available to any relevant interested parties. The LQM provides fundamental information to implement basic quality concepts principles and. Biohazard labels A symbol that must appear on all containers used to store waste products such as blood blood products or other specimens that may be infectious.
The quality manager should sit high in the organizational structure. Each laboratory must establish and follow written policies and procedures for a comprehensive quality assurance QA program designed to monitor and evaluate the ongoing and overall quality of the total testing process. This document provides guidance to public health laboratories on preparing a Laboratory Quality Manual.
The auditors chosen must have the technical skills needed to evaluate the area being audited and must have a good understanding of the laboratorys quality management system. Organization In order to have a functioning quality management system the structure and management of the laboratory must be organized so that quality policies can be established and implemented. It covers a wide range of matters that influence the.
The laboratory results must be as accurate as possible all aspects of the laboratory operations must be reliable and reporting must be timely in order.

Resume Sample Food Engineer Ile Ilgili Gorsel Sonucu Resume Examples Server Resume Sample Resume

Resume Format Quality Assurance Pharma Resumeformat
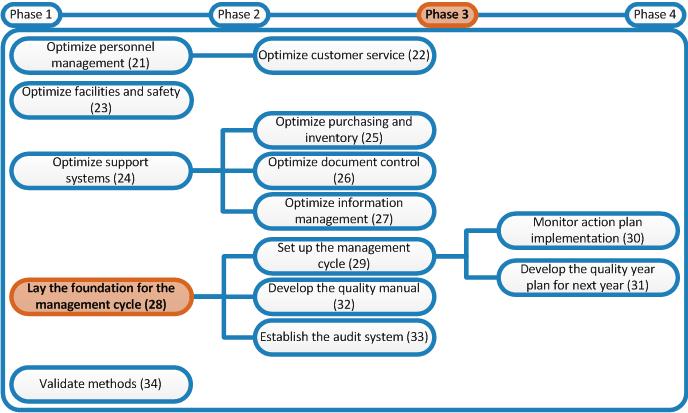
Laboratory Quality Stepwise Implementation Tool

Pdf Quality Assurance And Quality Control In Erp Systems Implementation

Quality Assurance In Medical Laboratories Paths To Global Competence Standards

Mortgage Quality Control Plan Template Inspirational Mortgage Quality Control Plan Template Clini How To Plan Lesson Plan Template Free Treatment Plan Template

Procedures And Good Laboratory Practices Glps Ppt Download
Pdf Chapter 4 Quality Assurance

Quality Assurance 1 Lecture 2 Dr Mazen Alzaharna

Quality Assurance 1 Lecture 2 Dr Mazen Alzaharna

Quality Assurance In The Clinical Laboratory Ppt Video Online Download

Resume Examples Quality Assurance Resume Templates Manager Resume Sales Resume Resume Examples

Quality Assurance 1 Lecture 2 Dr Mazen Alzaharna

Problem Action Result Resume Examples In 2021

9 Laboratory Quality Assurance Plan Examples In Pdf Examples

Quality Assurance In Laboratories Ppt Video Online Download

Quality Assurance In The Clinical Laboratory Ppt Video Online Download

Posting Komentar untuk "A Laboratory Must Have A Written Quality Assurance Policy That"