Quality Assurance Definition In Pharmacy
She has gained experience in Quality Assurance Quality Systems QA QS by completing work in both pharmacy and the food industry. Quality Assurance is one of the department in the pharmaceutical manufacturing industry.

Quality Assurance Training Pharmaceutical Industry Roles
Pharmaceutical quality assurance framework Defining and assessing pharmaceutical quality Consequences of poor pharmaceutical quality Determinants of pharmaceutical quality Prevalence of poor-quality pharmaceuticals Global quality-monitoring options.

Quality assurance definition in pharmacy. Although everyone in a company is ultimately responsible for quality executives and other members of top management have an important responsibility. Ensuring that pharmaceutical products are safe and effective is the primary goal of. Pharmacy Quality Assurance.
Quality Assurance in Industrial Pharmacy eg. Section A provides the context for quality assurance of. A unique and highly successful aspect of our program is the didactic component that all students receive industry standard work experience in our own GMP facility.
If asked most of us could come up with a definition of qualityBut quality can mean different things to different people and articulating the methods by which quality is achieved and maintained is not easy. Anastasia has worked in Greece in the food industry as a Quality Assurance technician and in the UK pharmaceutical industry at Norbrook Laboratories Ltd in Northern Ireland and gained experience in testing raw materials as a Quality Control Analyst. Quality assurance is a wide-ranging concept covering all matters that individually or collectively influence the quality of a product.
An alternate definition is the operational techniques and. Many areas within a health care system may be involved with quality assurance including procurement pharmacy medical and nursing departments as well as the DTC. Quality Assurance Department Functions in Pharmaceuticals.
Quality assurance is an important part of pharmaceutical manufacturing. This shall be achieved by performing the functions of monitoring as per the laid systems for the following areas which. With regard to pharmaceuticals quality assurance can be divided into major areas.
Responsibility for the definition evaluation and improvement of quality. A comprehensive quality assurance program includes both technical and managerial activities from selection to patient use. The pharmacist should be aware of essential medical and pharmaceutical information about each patient.
Table 12-1 reviews common terms associated with the quality assurance process that you may hear in your pharmacy. Quality control can be defined as part of quality management focused on fulfilling quality requirements While quality assurance relates to how a process is performed or how a product is made quality control is more the inspection aspect of quality management. 1Drug and narcotic control standards 2Drug industry standards.
Quality assurance of pharmaceuticals. 5 Foreword Purpose of the FIPEd Global Framework for Quality Assurance and the Intended Audience This document is an updated and expanded version of the FIP Global Framework for Quality Assurance of Pharmacy Education Version 1 adopted by the International Pharmaceutical Federation FIP in 2008 1It is presented in four sections. There should not be any compromise with quality.
Quality assurance in the health care field a pledge to the public by those within the various health disciplines that they will work toward the goal of an optimal achievable degree of excellence in the services rendered to every patient. WHO Library Cataloguing-in-Publication Data Quality assurance of pharmaceuticals. Quality-by-design QbD is defined in ICH Q8 guideline as a systematic approach with aim that the product quality and performance are achieved and assured by design of effective and efficient.
A drug that doesnt work as intended or that is defective in some way can present a threat to public health. 2 Good manufacturing practices and inspection. Appropriate quality assurance is important in the pharmaceutical industry.
Dubey Ne Gupta H Sharma RK Dubey Ni Dubey N 2011 Pharmaceutical Quality Management System. What Is The Definition Of Quality Assurance In Pharmaceuticals. Quality Assurance is assuring the quality of the drug product manufactured in the facility by implementing and following the compliance in the unit.
Development quality control production distribution and inspections. Since the 1960s there has been an increasing emphasis on the individual citizens right to health and the obligation. What does quality mean to you.
Outlines the benefits of a good Quality Management System QMS summarises key quality assurance. A compendium of guidelines and. We see assuring and encouraging quality improvement in pharmacy as an essential part of our role.
It is thought that documentation is the main function of quality assurance but it also controls the manufacturing system to. Pharmacy Management Volume 31 Issue 4 9 wwwpharmancouk A Quality Management System For Pharmacy Practice Titus De Silva Consultant in Pharmacy Practice Quality Management and Food Safety Email. Journal of Advanced Pharmacy Education Research 2.
Focuses on some aspects and need of maintaining Quality in Pharma through Quality Management System 1. Quality of the pharmaceutical must be the most important thing for pharmaceutical companies. Quality Assurance Department shall function for assuring the quality of all the Products manufactured at every stage of manufacturing processing of Drug Products.
A compendium of guidelines and related materials. It assures the quality of the products those are manufactured in manufacturing facility. Without it companies cannot guarantee that their products conform to the appropriate standards for quality and safety.
Quality Assurance Training Helps Ensure Drugs Work Safely and as Intended. Every person working in pharmaceuticals should always care about the product quality. The Pharmaceutical Quality Assurance Quality Control Diploma is awarded upon successful completion of all three modules.
Keep in mind that the voluntary accreditation processes through The Joint Commission and other organizations discussed in Chapter 3 Pharmacy Law and Regulation are part of pharmacys efforts to. CGMP ICH guidelines. A patient is safe effective and meets quality standards.

Differences Between Quality Assurance And Quality Control Pharmacy Youtube

Quality Assurance Pharmaceutical Quality Systems In Making Medicines

Quality Assurance Of Clinical Pharmacy Services
Quality Assurance Pharmaceutical Quality Systems In Making Medicines
Quality Assurance Pharmaceutical Quality Systems In Making Medicines

Browse 842 372 Work From Home Pharmacy Technician Jobs 29k 50k Hiring Now From Companies With Openings Find Your Next Jo Job Job Opportunities Job Posting
Quality Assurance Pharmaceutical Quality Systems In Making Medicines
Quality Control Of Pharmaceutical Products

Quality Metrics For Pharmaceutical Manufacturing Pharmaceutical Guidelines

The V Model Lifecycle For Managing Software Projects Software Projects Integration Testing Development
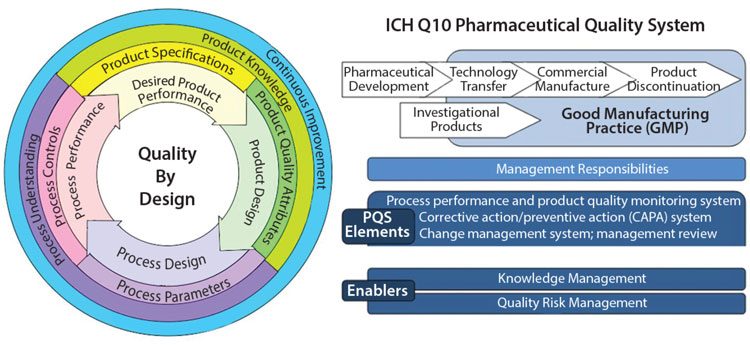
Quality Assurance Pharmaceutical Quality Systems In Making Medicines

Quality Assurance Training Pharmaceutical Industry Roles

Introduction To Quality Assurance And Qualtiy Control

Difference Between Quality Assurance And Quality Control Pharmaceutical Guidelines

Swot Analysis Of Pharmacy Swot Analysis Ideas Of Buying A House First Time Buyingahouse Housebuying Swo Swot Analysis Swot Analysis Template Analysis

Overview Of Validation Requirements Pharmaceutical Industry In 2021 Preventive Maintenance Pharmaceutical Pharmaceutical Industry

Pdf Review Of Quality Assurance In Hospital Pharmacy
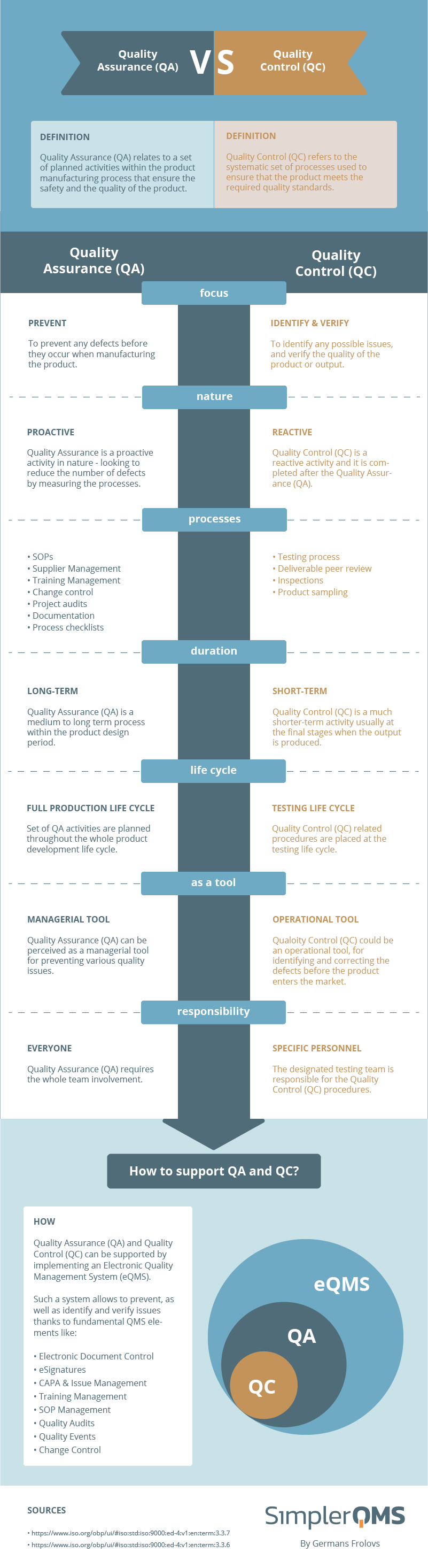
Quality Assurance Vs Quality Control Simplerqms

Posting Komentar untuk "Quality Assurance Definition In Pharmacy"